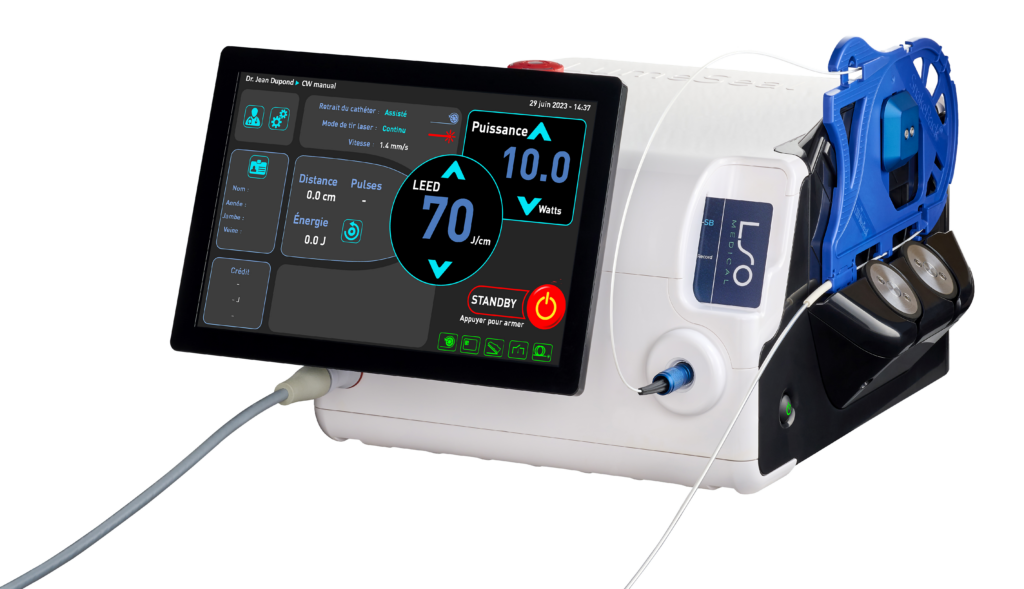
The 1470 nm endovenous laser for thermal ablation of varicose veins
- Occlusion of the great saphenous vein.
- Occlusion of the small saphenous vein.
Key features and compatible accessories
- Designed with SnakeBack® technology.
- Compatible with radial Ringlight_SB® fiber.
- Real-time auto-monitoring of the laser and fiber with the BackReflection™ system.
After the success of the Pharaoh 980 nm and following the validation of the clinical interest of the wavelength 1470 nm (Endotherme™ 1470), LSO Medical integrates a diode 1470 nm (infrared) in its new laser LumeSeal®.
This wavelength of 1470 nm combined with its “continuous” firing mode are now recognized as the Gold Standard in the thermal ablation treatment of saphenous by laser.
Since 2012, LSO Medical has been offering a complete range of Ringlight® radial fibers.
For added flexibility, Ringlight® radial fibers are available in 3 sizes:
- Standard 1.8 mm, 600 µm (+ option fused disponible)
- Slim Fused 1.3 mm, 400 μm
- Slim 1.0 mm, 400 μm
- Easy adjustment for better control of the procedure
- Assisted/automated fiber removal control managed by the user interface alone
The LumeSeal® incorporates the company’s latest patented innovation: SnakeBack® technology.
The SnakeBack® is a tension-free assisted removal system capable of planning, applying and controlling the delivery of energy into the vein.
The LumeSeal® thus allows a perfect delivery of energy, throughout the vein, maintaining a perfect regularity of treatment.
Complete traceability for each procedure:
- The LumeSeal® allows traceability of each procedure by the presence of a smart card delivered with each SB radial firing laser fiber.
- Laser: Laser Diode
- Wavelength: 1470 nm
- Laser class: 4
- Class DM: IIb (Directive 93/42/EEC and Regulation 2017/745/EU)
- Removal module: SnakeBack®
| Description | Reference | Technical specifications | Compatibility |
|---|---|---|---|
| RINGLIGHT® FIBER PROBE IR_SB 1,8 mm | ORLF000003_SB | Ringlight_SB® Fiber Standard 600 µm, 1.8 mm | Laser : LumeSeal® Introducer 6 Fr |
| RINGLIGHT® FIBER PROBE IRH_SB 1,0 mm | ORLF000005_SB | Ringlight_SB® Fiber Slim 400 µm, 1.0 mm | Laser : LumeSeal® Introducer 4 Fr or Cathlon 16G |
| RINGLIGHT® FIBER PROBE IR_SB 1,8 mm | ORLF000006_SB | Ringlight_SB® Fiber Fused Standard 600 µm, 1.8 mm | Laser : LumeSeal® Introducer 6 Fr |
| RINGLIGHT® FIBER PROBE IRH_SB 1,3 mm | ORLF000007_SB | Ringlight_SB® Fiber Fused Slim 400 µm, 1.3 mm | Laser : LumeSeal® Introducer 4 Fr or Cathlon 16G |
| Description | Reference | Technical specifications | Compatibility |
|---|---|---|---|
| Servante LumeSeal® | EV2-7-201 | Compatible LumeSeal_SB® | |
| Lunettes Praticiens | FG1#37 | Blue glasses lenses | |
| Lunettes Patients | IRD5#35 | Green glasses lenses |
| Description | Reference | Technical specifications | Compatibility |
|---|---|---|---|
| Dispenser DP 30 Pump – NOUVAG | 4187 |
| Nouvag 6022 / 6022a / 6022b tubing |
| Tubing – NOUVAG | 6022 / 6022a / 6022b | Length: 4 meters | Dispenser DP 30 Pump – NOUVAG |
| Vascular thermal ablation kit 6 Fr – LSO Medical | FR-K66189 | Ringlight® Fiber Standard | |
| Introduction kit 6 Fr – SCW | INTRO6F03818GS |
| Ringlight® Fiber Standard |
Intended user: vascular surgeons, vascular physicians, pharmacists, biomedical engineers, and operating room staff.
Medical laser equipment: LumeSeal_SB®. Medical device class: IIb (Directive 93/42/EEC and Regulation (EU) 2017/745). Laser class: 4 (protective eyewear required).
Sterile laser disposables: Ringlight® and Ringlight_SB®. Medical device class: IIa (Directive 93/42/EEC and Regulation (EU) 2017/745). Fibers are supplied sterile, sterilized with ethylene oxide, and intended for single use only. They must not be reused or resterilized.
![]() 1639
1639
Endovenous Laser Devices:
Intended Use
The LumeSeal_SB® laser device is used to produce laser energy with controlled parameters (notably wavelength, power, and emission mode) and to deliver it to a compatible output device, such as an endovenous optical fiber, to allow the controlled application of thermal energy within the target vein during endovenous procedures. The device is used under ultrasound guidance and in accordance with the manufacturer’s instructions for use.
Intended Purpose
The LumeSeal_SB® laser device is intended to generate and deliver laser energy for therapeutic purposes, for endovenous use in the coagulation, occlusion, and ablation of the saphenous veins of the lower limbs in patients with chronic venous disease, when endovenous thermal treatment is clinically indicated. The device is intended for use in adult patients by trained healthcare professionals in an appropriate medical environment.
Indications for Use
The LumeSeal_SB® laser device is indicated for use in procedures for the coagulation, occlusion, and endovenous thermal ablation of saphenous veins in patients presenting with venous insufficiency associated with documented venous reflux. The indication is based on a prior clinical and hemodynamic assessment, including an echo-Doppler examination, and on the practitioner’s decision in accordance with current clinical recommendations.
Sterile Consumables:
Intended Use
The Ringlight® / Ringlight_SB® endovenous optical fiber is used to deliver, diffuse, and apply laser energy in a controlled manner within the target vein during endovenous procedures, according to the parameters defined by the compatible laser device. The fiber is used under ultrasound guidance and in accordance with the manufacturer’s instructions for use.
Intended Purpose
The endovenous optical fiber is intended to transmit and diffuse laser energy, generated by a compatible laser device, for therapeutic purposes, for endovenous use in the coagulation, occlusion, and ablation of the saphenous veins of the lower limbs in patients with chronic venous disease, when endovenous thermal treatment is clinically indicated. The endovenous optical fiber is intended for use in adult patients by trained healthcare professionals in an appropriate medical environment.
Indications for Use
The endovenous optical fiber is indicated for use in procedures for the coagulation, occlusion, and endovenous thermal ablation of the saphenous vein in patients presenting with venous insufficiency associated with documented venous reflux. The indication is based on a prior clinical and hemodynamic assessment, including an echo-Doppler examination, and on the practitioner’s decision in accordance with current clinical recommendations.
Expected benefits: The advantages of endovenous laser treatment include: reduction in size or complete obliteration of treated varicose veins, and improvement or resolution of venous symptoms. The risk of varicose-related complications is significantly reduced.
Contraindications: LumeSeal_SB® must not be used in the following cases: Patients with thrombus in the vein segment to be treated, Patients with an aneurysm in the vein segment to be treated, Patients with peripheral vascular disease and an ankle–brachial index below 0.9, Patients with deep vein thrombosis (DVT) or a history of DVT, Pregnant patients, Patients with infection, active herpes, or other viral infection at the treatment site. Additional contraindications may be identified by the practitioner at the time of treatment.
Adverse effects: Based on published literature, three main categories of adverse events may occur during endovenous laser ablation of the great saphenous vein: frequent adverse events (incidence < 50%): bruising, pain, superficial vein thrombosis. Rare adverse events (incidence < 5% in most studies): deep vein thrombosis, paraesthesia, skin burn. Exceptional adverse events (fewer than 10 cases reported): permanent paraesthesia (beyond one year), pulmonary embolism.
Device use: Training in the use of LumeSeal® is provided by LSO Medical or by personnel authorised by LSO Medical. Carefully read the instructions provided in the user manual. To comply with performance requirements, the system must be calibrated at least once a year by an LSO Medical-approved technician or an authorised local distributor.
Medical device manufacturer: LSO Medical – Biocentre A. Fleming, Building D – 280 rue Salvador Allende – 59120 Loos, France
Distributor and importer: SCW, NOUVAG
Cleaning (plastic housing, touch screen): Use a damp cloth and a disinfectant detergent compliant with European microbiological standards: Bactericidal (EN 1040, EN 1276), Active against Aspergillus fumigatus and Mycobacterium tuberculosis, Fungicidal (EN 1275), Active against HIV-1, HBV, Rotavirus, Herpes virus, and BVDV (HCV substitute).
Disposal: LumeSeal® contains components requiring specific recycling. The product may be returned to the manufacturer or any authorised organisation.
Internal reference: 25/05/LSOMEDICAL/PM/001
Vigilance: Any serious incident related to the device must be reported to the manufacturer and to the competent authority of the Member State where the user or patient is established.


